A Unique RTB-Lectin-Based Delivery Platform
SylamoreBio’s proprietary technology, RTB, is a sugar-binding lectin that targets glycans abundantly expressed on human cell surfaces, enabling efficient, receptor-independent cellular uptake across diverse tissues. RTB enables targeted, corrective dosing in tissues historically resistant to therapeutic access including CNS, peripheral nerves, tumors, and beyond.
Key Advantages
Broad Cell Targeting: Leverages glycan recognition to mediate uptake across virtually all human cell types
Barrier Penetration: Successfully delivers cargo to challenging sites including the brain, and bone
Therapeutic Integrity: Maintains delivery efficiency even in the presence of anti-drug antibodies (ADA)
Versatile Payload Capability: Compatible with enzymes, antibodies, RNAs, and more
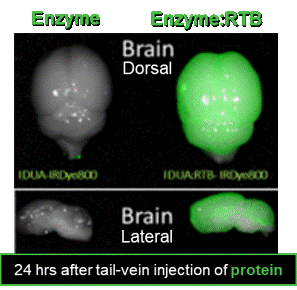
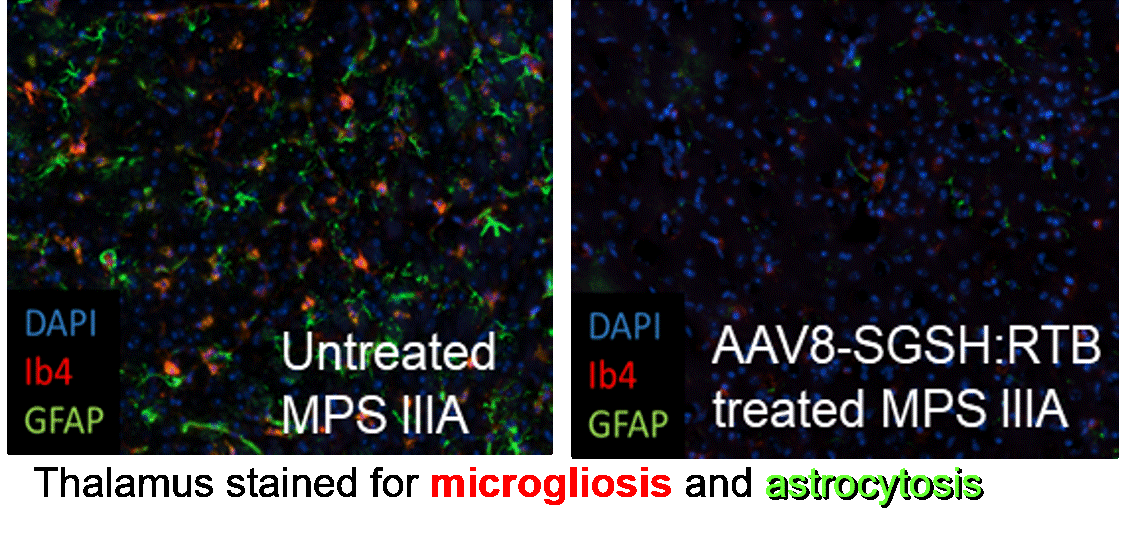
Targeting the CNS
AAV Liver-expressed enzyme:RTB normalizes CNS pathologies in multiple mouse models
Crosses BBB and penetrates throughout CNS
Clears disease substrate and corrects cellular pathology in CNS
Eliminates neuroinflammation linked with disease progression
Improves memory and learning benchmarks
Bone and Muscle Delivery
Delivery of therapeutics to bone and muscle is challenging. The avascular nature of cartilage in the growth plate limits the delivery of therapeutics for prevention of abnormal bone growth. Delivery to the muscle is also difficult and requires very high doses of product to achieve improvement due to the type of receptors present in muscle cells. Our lectin-fusion therapeutics are capable of delivering corrective doses of product to these tissues, proving to prevent the development of pathologies in animal models.
Mitigates the effect of anti-drug antibodies
Therapies design to replace the missing protein in lysosomal storage disorders are often compromised by the development of neutralizing antibodies to the enzyme (anti-drug antibodies; ADA). ADA responses hinder treatment efficacy of the majority of therapies available to patients, blocking the entry of the replacement therapeutic to key organs and tissues. Over 90% of the patients with Hurler (MPS I), MPS VIA, Pompe and Fabry develop antibodies to their approved drug.
Our lectin delivery platform is able to deliver corrective doses of enzyme to the brain, heart, kidney, bone and muscle even in the presence of blocking antibodies in animal models.